FLiPS
Using Dronpa to Activate and Monitor Protein Activity
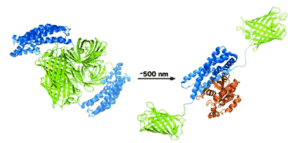
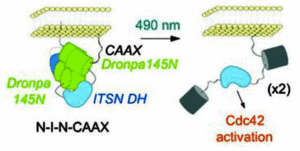
Dronpa, a fluorescent protein found in corals is multimeric and does not fluoresce in its monomeric state. Dronpa is usually green fluorescent, but when irradiated with 490nm light (cyan) it goes dark because the multimer falls apart. Michael Lin and his coworkers at Stanford University have used this property to design the first probe that both initiates and tracks enzyme activity. FliPs (Fluorescent Light induced Proteins) are created by attaching the proteins of interest to a Dronpa mutant. In its fluorescent tetrameric state the Dronpa complex cages the proteins and they cannot interact. Upon activation with cyan light the Dronpa tetramer falls apart, is no longer fluorescent and most importantly no longer prevents protein function, see figures below.