IR Fluorescent Proteins
To date our rainbow palette of GFP derived fluorescent proteins has been missing a bright far-red or infrared protein. Red light has the longest wavelength of all visible light, which means it has the lowest energy and causes the least amount of cellular damage. It also penetrates flesh the furthest as it is not absorbed by bodily materials like hemoglobin. Therefore many groups are trying to design red fluorescent proteins.
Not surprisingly the best red and infrared fluorescent proteins have come from the Tsien labs. In the May 8th 2009 issue of Science a new class of IFPs was described. They are based on bacterial phytochromes, not jellyfish GFPs. Bacterial phytochromes do not normally fluorescence, instead they absorb light and then use the energy obtained to send messages. However, if the components that send the message are removed from the phytochrome, the protein becomes fluorescent.
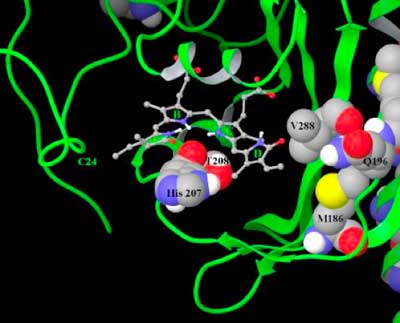
In order to fluoresce the bacterial phytochromes have to bind the tetrapyrrole shown on the right and the rotation of the D ring has to be restricted. The fluorescence quenching rotation was prevented by making some key mutations around the D ring (see Figure). Having to add the tetrapyrrole from an external source would severely limit the use the IFPs in in vivo imaging applications. Fortunately it is very common in the body, due to heme breakdown, and no additional source of the tetrapyrrole (biliverdin IXα) is required.