Structure
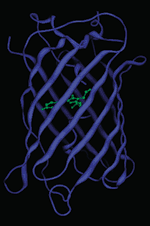
The crystal structure of GFP was solved in 1996. It has a unique soda can shape. Eleven beta-strands make up the beta-barrel and an alpha-helix runs through the center. The chromophore is located in the middle of the beta-barrel, it is occasionally referred to as the “light in the can.”
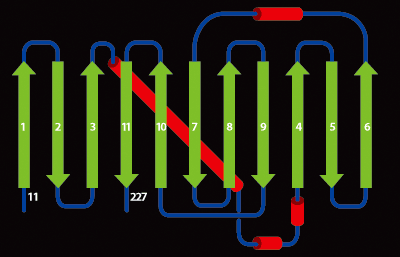
Another representation of the GFP beta-barrel. Beta sheets are green, helices red and connecting loops black.
In June 2003, GFP was the protein databank’s (pdb) molecule of the month.
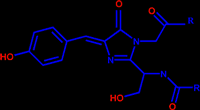
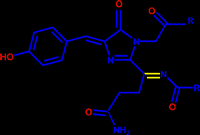
The chromophore of GFP is responsible for its fluorescence. It has the following structure where the R groups are the first 64 and last 170 residues of GFP. GFP catalyzes the formation of its own chromophore. It is proposed that Arg96 plays a crucial role in this catalysis. Two different mechanisms have been proposed for its action
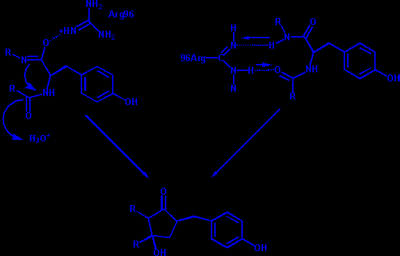
The biggest difference between green fluorescent protein and its red analog, DsRed, is that the chromophore of DsRed has an extra double bond (drawn in yellow) which extends the chromophores conjugation and causes the red-shift.