Highlighter Proteins:
FPs have been found and created that change their emission upon irradiation. They are known as optical highlighter. For convenience have been classified into three groups: Photoactivateable, Photoconvertable and Photoswitchable FPs.
Photoactivateable FPs
Photoactivateable FPs are dark and are irreversibly activated by irradiation. For example irradiation of PA-GFP with intense violet light results in a 100-fold increase in green fluorescence. It is presumed that the violet light causes the decarboxylation of Glu222, which aids in the formation of the anionic fluorescent form of the chromophore, see below.
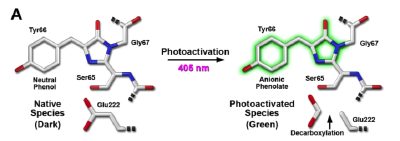

Photoactivation of mPA-GFP-actin in opossum kidney epithelial cells.
A) Circular region is irradiated with violet light (405nm) for 5 seconds.
B) After 5 minutes some of the activated mPA-GFP has diffused out of the circular area.
C) Sixty minutes after irradiation the ruffles, cyctoplasmic actin pools and filamentous actin networks have become brighter.
Photoconvertable FPs
can be irreversibly converted from a green fluorescent form to a red fluorescent form by violet or ultraviolet irradiation e.g. Kaede, KikGR, Dendra2 and Eos. The photoconversion is presumably associated with a cleavage occurring between the amide nitrogen and the alpha carbon of His62 that is followed by oxidation of the His62 sidechain.

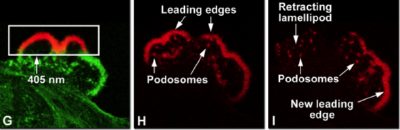
G) Rectangular area of Dendra2-actin labeled lamellipodia is photoconverted from green to red with 405nm radiation.
H) Using special filters only the photoconverted red Dendra2 is imaged 20 minutes after photoconversion.
I) Forty minutes later a new leading edge is observed.
Photoswitchable FPs
Photoswitchable FPs are dark and are reversibly activated by irradiation. Photoswithable FPs such as Dronpa, mTFP0.7 and KFP switch between the dark E (or trans) state and the fluorescent Z (or cis) state, see below.


J) Dronpa labeled actin network.
K) Dronpa labeled actin network switched off with 488nm irradiation before switching on the region spelling “FV10”
L) The opposite to K.
IrisFP In late 2008 a mutant of the photoconvertable FP Eos was reported. “Like its parent protein EosFP, IrisFP also photoconverts irreversibly to a red-emitting state under violet light because of an extension of the conjugated pi-cloud of the chromophore, accompanied by a cleavage of the polypeptide back-bone. The red form of IrisFP exhibits a second reversible photo-switching process, which may also involve cis-trans isomerization of the chromophore.” — Very Cool.